Abstract
Introduction: Secondary acute myeloid leukemia (AML) evolving in a patient who had a prior myeloproliferative neoplasm (MPN) is a challenging entity to treat, and associated with poor patient outcomes. The optimal approach to these patients is uncertain. In this study, we retrospectively assessed the outcomes of patients with an MPN evolving into AML, to determine factors that contribute to improved survival.
Methods: We retrospectively identified adult patients at Massachusetts General Hospital and Icahn School of Medicine at Mount Sinai, who had a preceding Ph-negative MPN diagnosis (polycythemia vera, PV; essential thrombocythemia, ET; myelofibrosis, MF) and who subsequently developed AML. We collected information about baseline characteristics, mutational data, treatment history, and transplant status. Overall survival (OS) was from the time of AML diagnosis until death; patients were otherwise censored at last known alive. Patient characteristics were reported descriptively and compared using the Fisher and log rank tests; OS was estimated by the method of Kaplan and Meier.
Results: We identified a total of 58 patients with an underlying MPN (PV n=14, ET n=26, MF n=13, other n=5) and subsequently diagnosed with AML between 2006-2016. The median age at the time of MPN diagnosis was 58 years, while the median age at AML diagnosis was 68 years (range 40-92). The median time from MPN diagnosis to AML was 9.1 years (range 0.3-36). More patients were male (59%), except those with prior ET (46% of subgroup). JAK2 status during MPN diagnosis was reported for 40 patients; 63% had a JAK2 V616F mutation (n=24) or exon 13 mutation (n=1). A total of 17 of 55 (31%) patients with transfusion history were transfusion dependent during their MPN diagnosis. Treatments for MPNs included hydroxyurea (n=38), anagrelide (n=9), lenalidomide (n=4), HMA (n=8), and ruxolitinib (n=11). A total of 2 patients underwent transplant for their MPN before progression to AML.
At the time of AML transformation, 2 of 33 tested patients had FLT3-ITD mutations, both with prior ET. Patients had intermediate (37%) and poor risk (63%) karyotype at AML diagnosis. 2 patients had mutated NPM1 and 1 had mutated CEBPA . Of 44 patients with mutated JAK2 testing at AML diagnosis, 64% had a JAK2 V617F (n=27) or exon 13 (n=1) mutation. For patients with testing at both MPN and AML diagnosis (n=34), 1 patient had a newly identified JAK2 V617F mutation at AML diagnosis, while 2 patients with a JAK2- mutated MPN were wildtype at AML diagnosis. Upon transformation to AML, patients received induction chemotherapy (n=14), HMA (n=27), other therapy (n=5), went direct to transplant (n=2) or received supportive care alone (n=9); 1 patient did not have AML treatment history available. More patients achieved remission with induction (64%) than HMA (15%). Median OS from AML transformation was 5.8 months for the entire group, and of the 14 patients achieving CR, PFS was 14.9 months. Median OS varied per AML therapy: induction (8.3 mo), HMA (6.6), other therapy (8.8) and supportive care (1.4). There was no impact on whether a patient had received ruxolitinib previously for their MPN and on survival outcomes (p=0.28). Patients with TP53 mutations (n=3) had worse survival compared to WT (n=14, p=0.0279). Among all patients, median OS was better for those who achieved CR (not reached, p=0.0016). Patients with transfusion dependence were less likely to achieve CR (6% CR) and had worse OS compared to non-transfusion-dependent (median 7.3 vs 3.7 mo, p=0.033). Among patients not undergoing HCT, OS at 1 year post-AML transformation was 21% and median OS was 3.8 months.
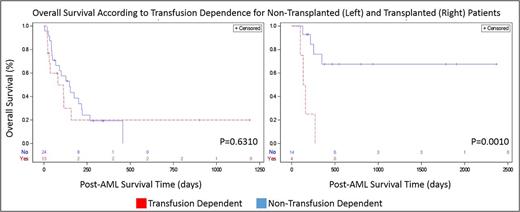
A total of 19 patients underwent transplant for AML, including 10/13 patients who achieved CR. At 1-y post-HCT, 54% of patients were alive. Patients who were in CR prior to transplant did not have a significantly improved OS compared to those without CR (p=0.30); however, those patients who were transfusion dependent prior to HCT had worse OS compared to non-transfusion dependent patients (Figure 1; median OS not reached vs. 4.mo, p=0.0010).
Discussion: AML progression from a prior MPN represents an unmet clinical need. Survival is improved among patients who achieve a CR to AML therapy and those receiving transplant. In this study we also find that prior transfusion dependence during the MPN diagnosis is associated with poor OS among patients undergoing transplant, and may be a consideration in the pre-HCT evaluation.
Brunner: Celgene: Research Funding; Takeda: Research Funding. Mascarenhas: CTI Biopharma: Research Funding; Merck: Research Funding; Janssen: Research Funding; Promedior: Research Funding; Novartis: Other: DSMB member , Research Funding; Incyte: Other: Clinical Trial Steering Committee , Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal